21 2 Naming Complex Ions And Coordination Compounds General Chemistry

Class 12 Chemistry Coordination Compounds Pdf Coordination Complex Ligand Chad breaks down how to name complex ions (both cations and anions) and coordination complexes. Chad provides a thorough lesson on how to name complex ions and coordination compounds.

21 Complex Ions Coordination Compounds Chemistry Libretexts These are homework exercises to accompany the textmap created for "general chemistry: principles and modern applications " by petrucci et al. A complex can be an anion, a cation ion, or a neutral molecule. coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex. Practice naming entire complex ions & coordination compounds learn with flashcards, games, and more — for free. Chadsprep 68 21.2 nomenclature of complex ions and coordination compounds rules for naming complex ions and coordination compounds 1) name the cation as the first word, then the anion as the second word in a coordination compound. neutral complexes are named using a single word.
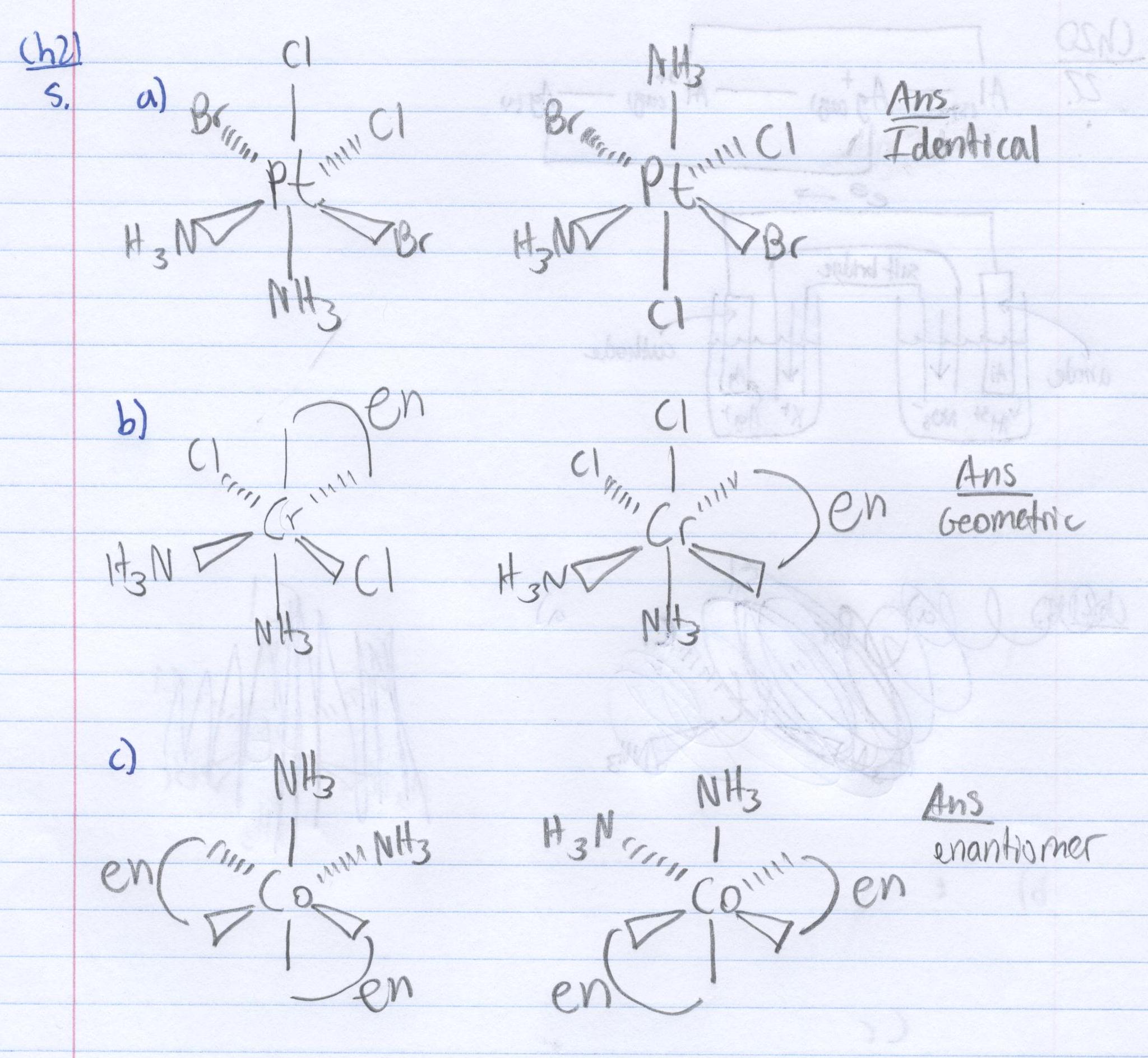
21 Complex Ions Coordination Compounds Chemistry Libretexts Practice naming entire complex ions & coordination compounds learn with flashcards, games, and more — for free. Chadsprep 68 21.2 nomenclature of complex ions and coordination compounds rules for naming complex ions and coordination compounds 1) name the cation as the first word, then the anion as the second word in a coordination compound. neutral complexes are named using a single word. You will notice that the nomenclature has not changed much from what we learned about transition metal nomenclature, you name the metal ion, indicate its charge with a roman numeral, and then add the anion onto the end. Next up in our discussion of section 18 transition metals and coordination chemistry, we'll cover naming coordination compounds and isomerism of complex ions. To begin naming coordination complexes, here are some things to keep in mind. ligands are named first in alphabetical order. the name of the metal comes next. the oxidation state of the metal follows, noted by a roman numeral in parentheses (ii, iv). These naming conventions are crucial for accurately identifying complex ions in coordination chemistry. understanding the distinction between standard and latin based names allows for clearer communication and comprehension of chemical compounds.

21 Complex Ions Coordination Compounds Chemistry Libretexts You will notice that the nomenclature has not changed much from what we learned about transition metal nomenclature, you name the metal ion, indicate its charge with a roman numeral, and then add the anion onto the end. Next up in our discussion of section 18 transition metals and coordination chemistry, we'll cover naming coordination compounds and isomerism of complex ions. To begin naming coordination complexes, here are some things to keep in mind. ligands are named first in alphabetical order. the name of the metal comes next. the oxidation state of the metal follows, noted by a roman numeral in parentheses (ii, iv). These naming conventions are crucial for accurately identifying complex ions in coordination chemistry. understanding the distinction between standard and latin based names allows for clearer communication and comprehension of chemical compounds.
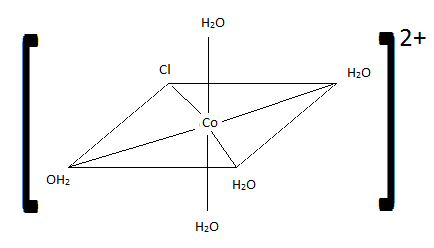
21 Complex Ions Coordination Compounds Chemistry Libretexts To begin naming coordination complexes, here are some things to keep in mind. ligands are named first in alphabetical order. the name of the metal comes next. the oxidation state of the metal follows, noted by a roman numeral in parentheses (ii, iv). These naming conventions are crucial for accurately identifying complex ions in coordination chemistry. understanding the distinction between standard and latin based names allows for clearer communication and comprehension of chemical compounds.
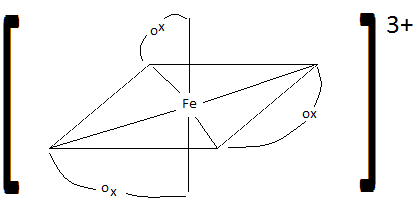
21 Complex Ions Coordination Compounds Chemistry Libretexts
Comments are closed.