Bond Order Length And Energy

Bond Order Length And Energy Bond Order The
Bond Order Length And Energy Bond Order The Bond strengths increase as bond order increases, while bond distances decrease. bond energy is defined as the energy required to break a particular bond in a molecule in the gas phase. its value depends on not only the identity of the bonded atoms but also their environment. Bond length is influenced primarily by the bond order, which is the number of shared electron pairs between two atoms. it is typically measured in picometers (pm) or angstroms (Å).

Bond Order Length And Energy Bond Order The
Bond Order Length And Energy Bond Order The Bond order: by definition, it is the number of shared electron pairs involved in the bond. it can also be looked at from the view of molecular orbital theory, where sometimes the order is calculated as half of the number of electrons that are bonded minus antibonding. For a covalent compound, the bond order and bond energy term provides us information on the number of bonds and the energy change when the bond is made or broken. When bond order is increased, bond length is decreased. atoms. in a covalent compound, a single bond has a bond order of one, a. three, and so on. 1. 2. draw the lewis structure. figure out the type of bond between the two atoms. example: determine the bond order for cyanide: cn . 1) draw the lewis structure. Several bond parameters, such as bond length, bond angle, bond order, and bond energy, can be used to characterize covalent bonds (also known as bond enthalpy). these bond parameters provide information about the stability of a chemical compound as well as the strength of the chemical bonds that hold its atoms together. what is a bond?.

Bond Length And Bond Energy
Bond Length And Bond Energy When bond order is increased, bond length is decreased. atoms. in a covalent compound, a single bond has a bond order of one, a. three, and so on. 1. 2. draw the lewis structure. figure out the type of bond between the two atoms. example: determine the bond order for cyanide: cn . 1) draw the lewis structure. Several bond parameters, such as bond length, bond angle, bond order, and bond energy, can be used to characterize covalent bonds (also known as bond enthalpy). these bond parameters provide information about the stability of a chemical compound as well as the strength of the chemical bonds that hold its atoms together. what is a bond?. Covalent bonds can be characterized on the basis of several bond parameters such as bond length, bond angle, bond order, and bond energy (also known as bond enthalpy). Coulomb's law states that the bond energy is inversely related to the bond length (r), and so factors which influence a bond's strength influence its length. this can allow us to determine some trends in bond lengths. Since we measure bond distance (length) from nucleus to nucleus, it makes sense that bigger atoms have longer bond lengths and that is true. but there are some trends and patterns that one should learn, memorize, and take note of. bond order is just a way for us to put a "scale" on bond types. Determining exact bond length is a matter of experiment, but estimating relative bond length is possible from trends in atomic size and bond order. we can always think of energy in terms of work. bond energy is the amount of work we need to do to pull the atoms in the bond completely apart.

Bond Length And Bond Energy
Bond Length And Bond Energy Covalent bonds can be characterized on the basis of several bond parameters such as bond length, bond angle, bond order, and bond energy (also known as bond enthalpy). Coulomb's law states that the bond energy is inversely related to the bond length (r), and so factors which influence a bond's strength influence its length. this can allow us to determine some trends in bond lengths. Since we measure bond distance (length) from nucleus to nucleus, it makes sense that bigger atoms have longer bond lengths and that is true. but there are some trends and patterns that one should learn, memorize, and take note of. bond order is just a way for us to put a "scale" on bond types. Determining exact bond length is a matter of experiment, but estimating relative bond length is possible from trends in atomic size and bond order. we can always think of energy in terms of work. bond energy is the amount of work we need to do to pull the atoms in the bond completely apart.
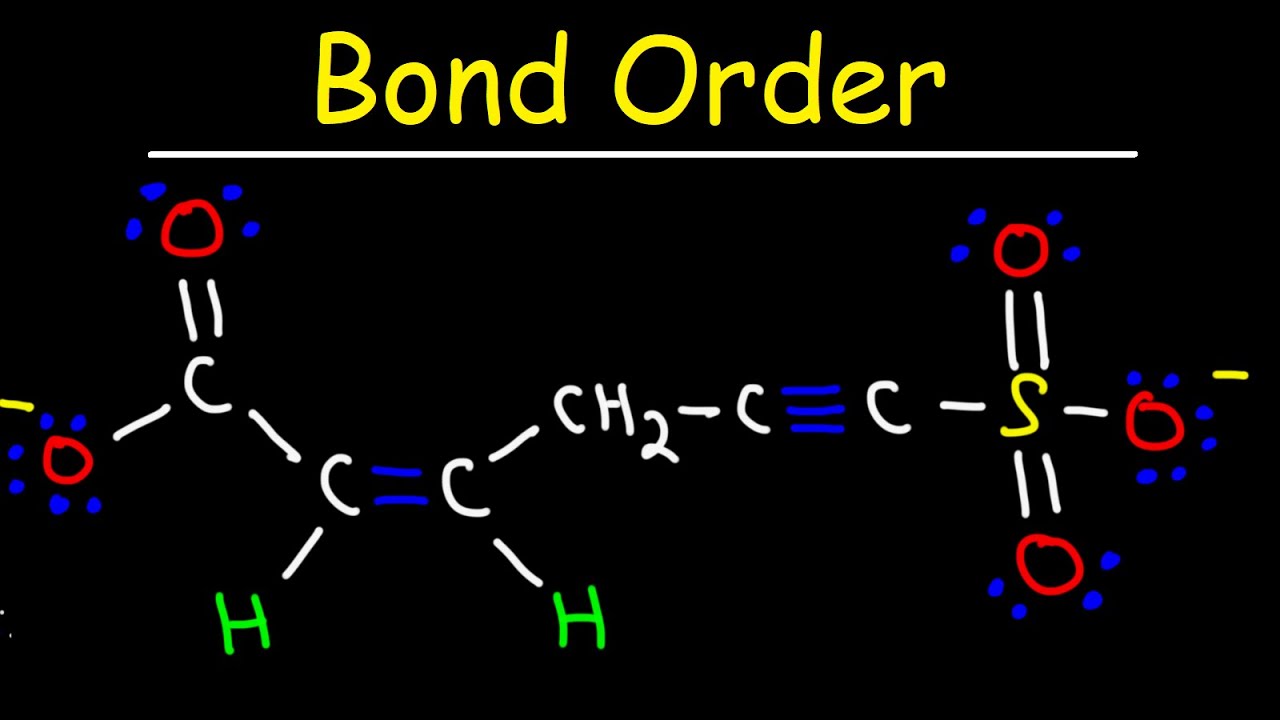
Bond Order and Resonance Structures
Bond Order and Resonance Structures
Related image with bond order length and energy
Related image with bond order length and energy
About "Bond Order Length And Energy"















Comments are closed.