Difference Between True Solution Suspension And Colloidal Sol

Difference Between True Solution Suspension And Colloidal Sol Edurev Class 9 Question True solutions are the type of mixtures, where the solute and solvents are properly mixed in the liquid phase. colloidal solutions are the type of mixture, where the solute (tiny particles or colloids) is uniformly distributed in the solvent (liquid phase). Difference between true solution, suspension and colloidal solution the table given below summarizes the major properties and points of distinction between each type of solution with respect to different properties.

Difference Between True Solution Colloidal Solution And Suspension With Comparison Chart What is the difference between solution, suspension and colloids? ca maninder singh is a chartered accountant for the past 14 years and a teacher from the past 18 years. he teaches science, economics, accounting and english at teachoo. Learn how colloidal solutions, true solutions, and suspensions vary in physical and chemical properties based on their size, appearance, and chemical reactions. The main difference between solutions, suspensions, and colloids is the difference in their particle size. the particles in solutions dissolve properly in the solvent, while suspensions and colloids contain dispersed particles. Based on the nature of particle size, appearance and filterability solutions are classified into three main categories, namely (1) true solution, (2) colloidal solution and (3) suspension.

Difference Between Suspension And Colloidal Solution Sinaumedia The main difference between solutions, suspensions, and colloids is the difference in their particle size. the particles in solutions dissolve properly in the solvent, while suspensions and colloids contain dispersed particles. Based on the nature of particle size, appearance and filterability solutions are classified into three main categories, namely (1) true solution, (2) colloidal solution and (3) suspension. In true solutions, the solute is completely dissolved in the solvent at the molecular level, whereas in colloidal solutions, the solute particles are dispersed but not dissolved, and in suspensions, the solute particles are suspended and not dissolved at all. In different physical and chemical procedures, all three solutions have variable characteristics and properties, and the significant difference lies in the particle size, appearance, and separation procedure. Difference between true solution, suspension and colloidal sol? the true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. In a true solution, the particle size ranges from 0.1 to 1 nm. a colloidal solution, or colloidal suspension, is a heterogeneous mixture in which particle size of substance is intermediate (between 1 1000 nm). the substance particles are evenly distributed within other substance.
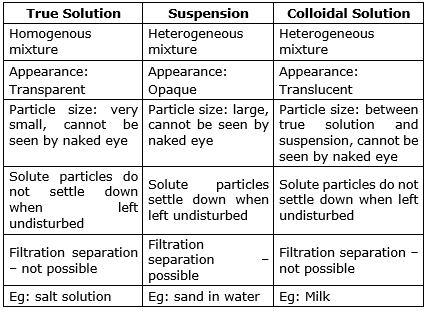
Differentiate Between True Solution Colloidal Solution And Suspension In true solutions, the solute is completely dissolved in the solvent at the molecular level, whereas in colloidal solutions, the solute particles are dispersed but not dissolved, and in suspensions, the solute particles are suspended and not dissolved at all. In different physical and chemical procedures, all three solutions have variable characteristics and properties, and the significant difference lies in the particle size, appearance, and separation procedure. Difference between true solution, suspension and colloidal sol? the true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. In a true solution, the particle size ranges from 0.1 to 1 nm. a colloidal solution, or colloidal suspension, is a heterogeneous mixture in which particle size of substance is intermediate (between 1 1000 nm). the substance particles are evenly distributed within other substance.

Difference Between True Solution And Colloidal Solution Definition Properties Examples Difference between true solution, suspension and colloidal sol? the true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. In a true solution, the particle size ranges from 0.1 to 1 nm. a colloidal solution, or colloidal suspension, is a heterogeneous mixture in which particle size of substance is intermediate (between 1 1000 nm). the substance particles are evenly distributed within other substance.
Comments are closed.