Energies E1 E2 And E3 Respectively The Energies Of Th Structures Fol

Energies E1 ,E2 And E3 , Respectively. The Energies Of Th Structures Fol..
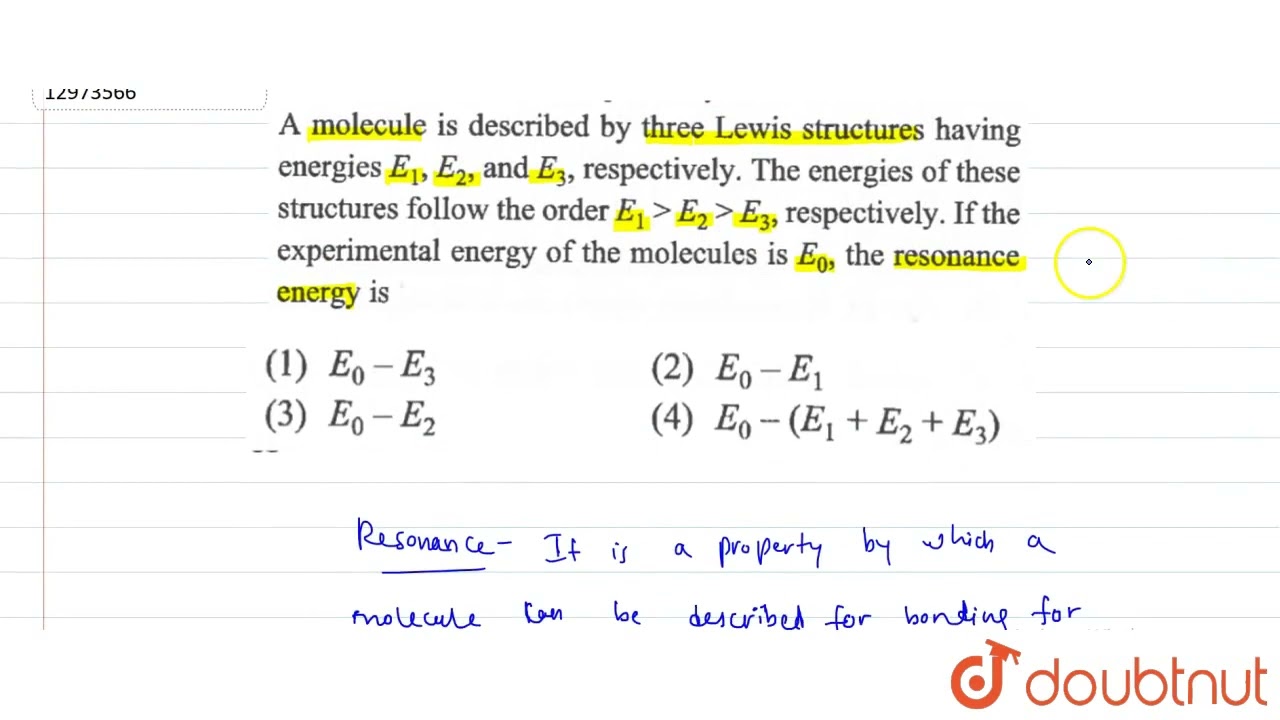
Energies E1 ,E2 And E3 , Respectively. The Energies Of Th Structures Fol.. To find the resonance energy of the molecule described by the three lewis structures with energies e1, e2, and e3, we can follow these steps: resonance refers to the phenomenon where a molecule can be represented by two or more valid lewis structures (canonical forms) that differ only in the arrangement of electrons. The correct answer is resonance energy is difference of most stable resonating energy and energy of real molecule.

A Molecule May Be Represented By Three Structures Having Energies E1, E2 And E3 Respectively ...
A Molecule May Be Represented By Three Structures Having Energies E1, E2 And E3 Respectively ... So we are going to discuss what is the correct order for the energy when we calculate resonance energy. Given the same de broglie wavelength for an electron, an α particle, and a proton, the kinetic energies are dictated by their masses due to the inverse relationship in de broglie's hypothesis. the correct energy order is α particle > proton > electron (e₂>e₃>e₁), making the correct choice b. Energies e1 ,e2 and e3 , respectively. the energies of th structures fol cbse chemistry. Resonance stabilizes the molecule or ion as the energy of the resonance hybrid (actual structure of the molecule or ion) is less than the energy of any single cannonical structure.

SOLVED:A Molecule May Be Represented By Three Structures Having Energies E1, E2 And E3 ...
SOLVED:A Molecule May Be Represented By Three Structures Having Energies E1, E2 And E3 ... Energies e1 ,e2 and e3 , respectively. the energies of th structures fol cbse chemistry. Resonance stabilizes the molecule or ion as the energy of the resonance hybrid (actual structure of the molecule or ion) is less than the energy of any single cannonical structure. Resonance stabilizes the molecule or ion as the energy of the resonance hybrid (actual structure of the molecule or ion) is less than the energy of any single cannonical structure. The correct answer is resonance energy is defined as difference between experimental bond energy of the molecule (e0) and energy of most stable resonating structures (e8). r.e. =e0−e3. If e1, e2 and e3 represent respectively the kinetic energies of an electron, an alpha particle and a proton, each having same de broglie wavelength then?. If e1, e2, and e3 represent respectively the kinetic energies of an electron, an alpha particle, and a proton, each having the same de broglie wavelength, then which of the following is correct? 1) e1> e3> e2 2) e2>e3> e1 3) e1> e2> e3 4) e1 = e2= e3.

A Molecule May Be Represented By Three Structures Energies E1 ,E2 And E3..
A Molecule May Be Represented By Three Structures Energies E1 ,E2 And E3.. Resonance stabilizes the molecule or ion as the energy of the resonance hybrid (actual structure of the molecule or ion) is less than the energy of any single cannonical structure. The correct answer is resonance energy is defined as difference between experimental bond energy of the molecule (e0) and energy of most stable resonating structures (e8). r.e. =e0−e3. If e1, e2 and e3 represent respectively the kinetic energies of an electron, an alpha particle and a proton, each having same de broglie wavelength then?. If e1, e2, and e3 represent respectively the kinetic energies of an electron, an alpha particle, and a proton, each having the same de broglie wavelength, then which of the following is correct? 1) e1> e3> e2 2) e2>e3> e1 3) e1> e2> e3 4) e1 = e2= e3.
Compare The Energies, E1 And E2 Respectively Of Two Radiations, One Wit..
Compare The Energies, E1 And E2 Respectively Of Two Radiations, One Wit.. If e1, e2 and e3 represent respectively the kinetic energies of an electron, an alpha particle and a proton, each having same de broglie wavelength then?. If e1, e2, and e3 represent respectively the kinetic energies of an electron, an alpha particle, and a proton, each having the same de broglie wavelength, then which of the following is correct? 1) e1> e3> e2 2) e2>e3> e1 3) e1> e2> e3 4) e1 = e2= e3.
A molecule is described by three Lewis structures having energies E_1, E_2, and E_3, respectivel...
A molecule is described by three Lewis structures having energies E_1, E_2, and E_3, respectivel...
Related image with energies e1 e2 and e3 respectively the energies of th structures fol
Related image with energies e1 e2 and e3 respectively the energies of th structures fol
About "Energies E1 E2 And E3 Respectively The Energies Of Th Structures Fol"














Comments are closed.