Fda Updates Labeling For Dolutegravir Rilpivirine Infectious Disease
Dolutegravir Rilpivirine Tablet Film Coated Nih Juluca, a two drug combination of dolutegravir, a human immunodeficiency virus type 1 (hiv 1) integrase strand transfer inhibitor (insti), and rilpivirine, an hiv 1 non nucleoside reverse. The food and drug administration (fda) has approved an update to the labeling for juluca (dolutegravir rilpivirine; janssen) to correct dosing separation between juluca and oral calcium and iron.
Dolutegravir Effectively Suppresses Hiv In Pregnant Women To update use in specific populations, pregnancy section with updated information regarding the prevalence of neural tube defects among infants born to individuals taking dolutegravir in the. Please refer to your supplemental new drug application (snda) dated and received april 7, 2022, and your amendments, submitted under section 505(b) of the federal food, drug, and cosmetic act. Dolutegravir tablets is indicated in combination with rilpivirine as a complete regimen for the treatment of hiv 1 infection in adults to replace the current antiretroviral regimen in those who. Virologically suppressed subjects the ars observed for dolutegravir tablets plus rilpivirine in the week 48 analysis of pooled data from two identical, international, multicenter, open label.
Fda Oks First Dispersible Form Of Dolutegravir For Infants Dolutegravir tablets is indicated in combination with rilpivirine as a complete regimen for the treatment of hiv 1 infection in adults to replace the current antiretroviral regimen in those who. Virologically suppressed subjects the ars observed for dolutegravir tablets plus rilpivirine in the week 48 analysis of pooled data from two identical, international, multicenter, open label. 8 labeling results fdalabel, nctr drug label search application. Juluca, a two drug combination of dolutegravir, a human immunodeficiency virus type 1 (hiv 1) integrase strand transfer inhibitor (insti), and rilpivirine, a hiv 1 non nucleoside reverse transcriptase inhibitor (nnrti), is indicated as a complete regimen for the treatment of hiv 1 infection in adults to replace the current antiretroviral. Advise the patient to read the fda approved patient labeling (patient information). severe skin and hypersensitivity reactions advise patients to immediately contact their healthcare provider if.
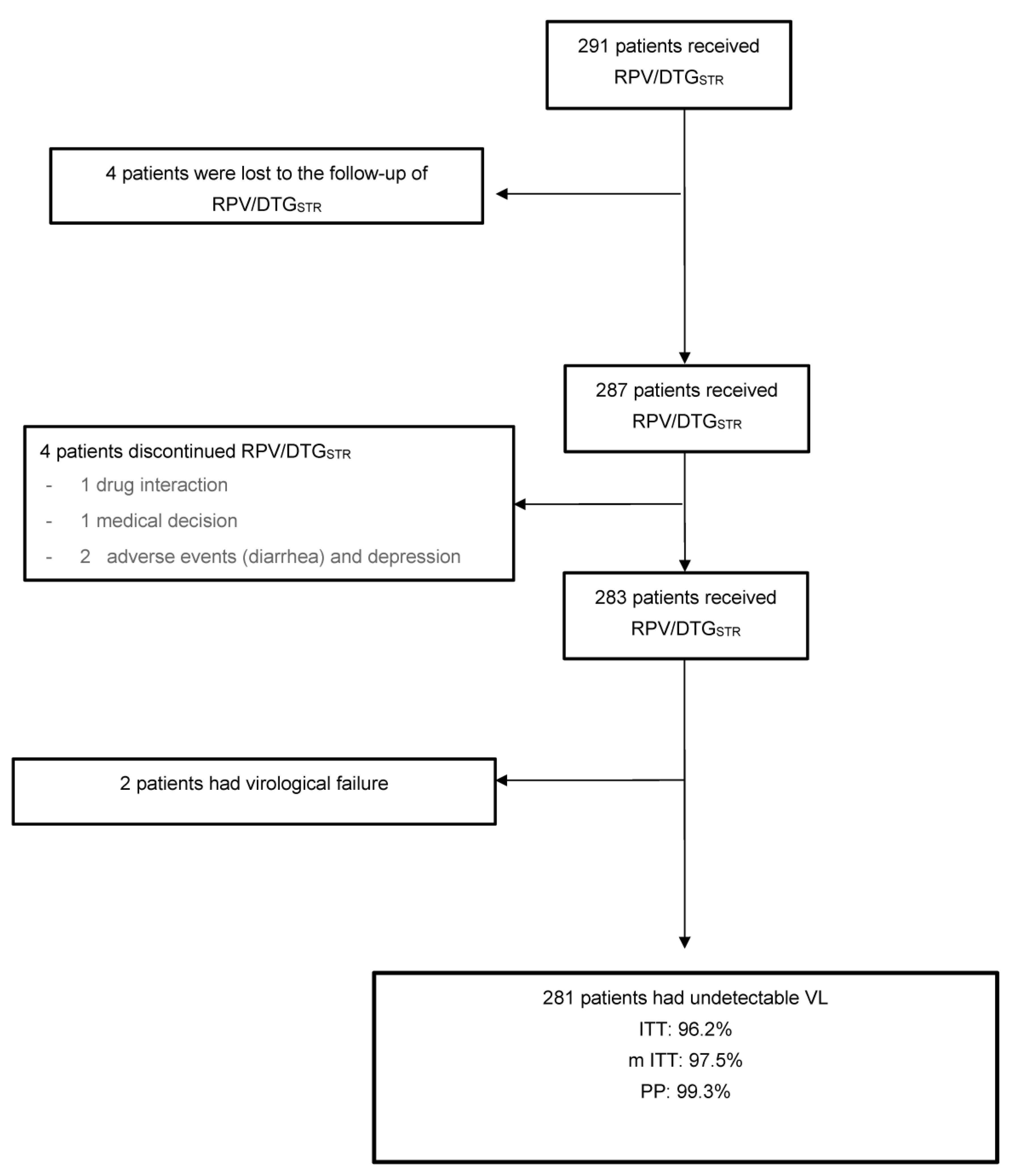
Viruses Free Full Text Rildo Real World Multicenter Study On The Effectiveness And Safety 8 labeling results fdalabel, nctr drug label search application. Juluca, a two drug combination of dolutegravir, a human immunodeficiency virus type 1 (hiv 1) integrase strand transfer inhibitor (insti), and rilpivirine, a hiv 1 non nucleoside reverse transcriptase inhibitor (nnrti), is indicated as a complete regimen for the treatment of hiv 1 infection in adults to replace the current antiretroviral. Advise the patient to read the fda approved patient labeling (patient information). severe skin and hypersensitivity reactions advise patients to immediately contact their healthcare provider if.
Comments are closed.