Pdf Liquid Biopsy Testing For The Management Of Patient With Non

Liquid Biopsy Download Free Pdf Micro Rna Biopsy Here, we show how the molecular tumor board (mtb) in our cancer center employed liquid biopsy to support therapeutic decisions in a patient with nsclc carrying a rare egfr mutation. Several liquid biopsy tests have been designated by the food and drug administration (fda) as companion diagnostic (cdx) assays deemed necessary for the effective use of a specific medication in the context of a specific clinical indication.

Pdf Liquid Biopsy Testing For The Management Of Patient With Non Small Cell Lung Cancer Tissue samples are often too small to get patients complete genomic testing • this can lead to patients being given less effective therapies. Liquid biopsy may be used to identify ntrk1 2 3 fusions in patients with solid tumors.12 they can also detect ret fusions in patients with solid tumors.13 patients with solid tumors who have these gene fusions may benefit from targeted therapy. Here, we show how the molecular tumor board (mtb) in our cancer center employed liquid biopsy to support therapeutic decisions in a patient with nsclc carrying a rare egfr mutation. Our study compared patients who also underwent liquid biopsy with patients who did not, in order to determine whether performance of this biopsy affected their treatment.
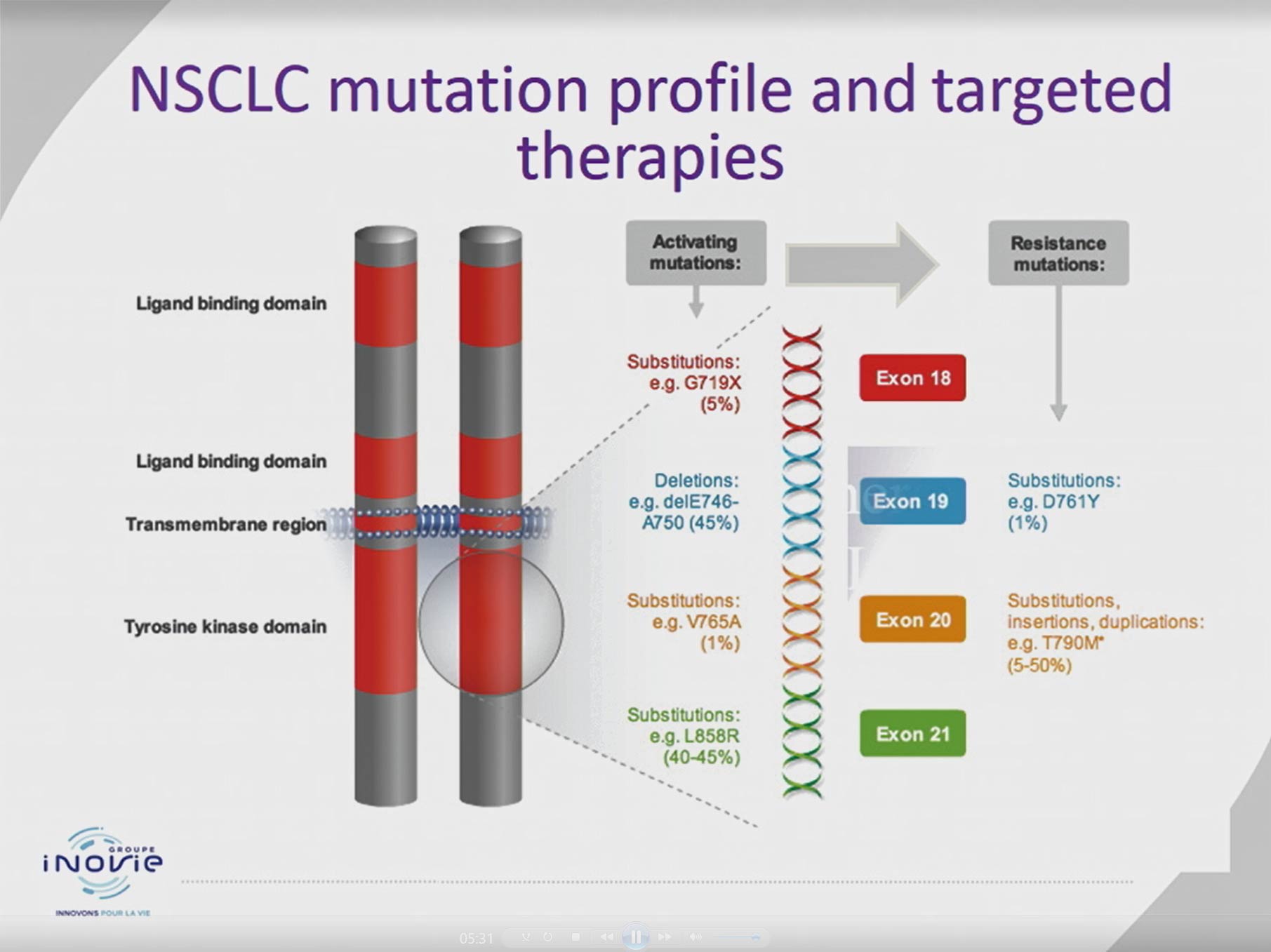
Disease Monitoring With Cfdna Liquid Biopsy Solutions From Agena Bioscience Here, we show how the molecular tumor board (mtb) in our cancer center employed liquid biopsy to support therapeutic decisions in a patient with nsclc carrying a rare egfr mutation. Our study compared patients who also underwent liquid biopsy with patients who did not, in order to determine whether performance of this biopsy affected their treatment. We carried out clinical validation of ctdna testing as liquid biopsy in patients with advanced nsclc in iso15189 accredited biopathology laboratory. The clinical applications of liquid biopsy in cancer management represent a significant advancement in oncology, providing non invasive methods for early detection, diagnosis, treatment monitoring, and personalized care. The liquid biopsy ngs assay demonstrated excellent concordance with tissue profiling and its use led to the detection of 26% more actionable alterations compared with standard of care tissue testing (pritchett). Liquid biopsy based cell free tumor dna (ctdna) may detect and allow matching and monitoring patients’ genomic alterations to specific therapies. in the clinics, ctdna has been proven to guide therapy in non small cell lung, prostate, ovarian, and breast cancer patients.
Comments are closed.