Solved 4 The Mole Learning Goal Use Avogadro S Number To Chegg
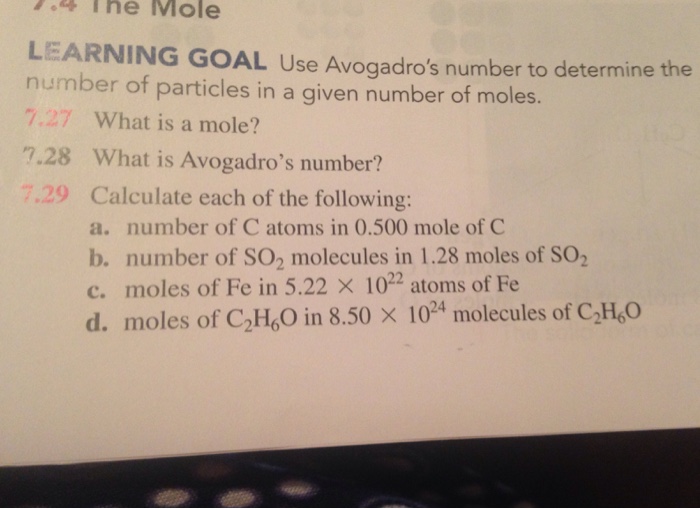
Solved 4 The Mole Learning Goal Use Avogadro S Number To Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. Use avogadro's number to convert to moles and vice versa given the number of particles of an element. know the definition of the mole. determine the formula mass of an ionic or molecular compound. determine the percent composition of each element in a compound from the chemical formula.

Solved Learning Goal To Use Avogadro S Number To Convert Chegg The relationships atomic mass, the mole, and avogadro’s number can be applied to compute various quantities that describe the composition of an elemental substance. To calculate the number of atoms in a sample of a given mass, we divide by the molar mass and multiply by avogadro's number. we can see that using dimensional analysis, units of grams and moles will be cancelled out, and the desired units of atoms are left in the equation. The mole is a unit of measure that corresponds to a group of 6.022 x 10e23 particles. this number of particles corresponds to avogadro's number. The number will of course depend both on the formula of the substance and on the weight of the sample. however, if we consider a weight of substance that is the same as its formula (molecular) weight expressed in grams, we have only one number to know: avogadro's number.

Solved Learning Goal To Use Avogadro S Number To Convert Chegg The mole is a unit of measure that corresponds to a group of 6.022 x 10e23 particles. this number of particles corresponds to avogadro's number. The number will of course depend both on the formula of the substance and on the weight of the sample. however, if we consider a weight of substance that is the same as its formula (molecular) weight expressed in grams, we have only one number to know: avogadro's number. Learning goal: to use avogadro's number to convert between microscopic units of atoms and molecules to the macroscopic world of chemical experiments. a mole is a unit of matter that represents a known number of particles that is large enough to be weighed on a laboratory balance. Learning objectives use avogadro's number to convert to moles and vice versa given the number of particles of an element. use the molar mass to convert to grams and vice versa given the number of moles of an element. Here’s the best way to solve it. multiply the number of moles of l i by avogadro's number to find the number of l i atoms. 7.1 one mole of a given substance is defined as the amount of substance w …. Use molar masses to convert masses to moles, and use avogadro's number (6.02×1023 particles per mole) to convert number of particles to moles. next, convert moles of the given reactant or product to moles of the desired reactant or product using the coefficients of the balanced chemical equation.

Solved х Learning Goal To Use Avogadro S Number To Convert Chegg Learning goal: to use avogadro's number to convert between microscopic units of atoms and molecules to the macroscopic world of chemical experiments. a mole is a unit of matter that represents a known number of particles that is large enough to be weighed on a laboratory balance. Learning objectives use avogadro's number to convert to moles and vice versa given the number of particles of an element. use the molar mass to convert to grams and vice versa given the number of moles of an element. Here’s the best way to solve it. multiply the number of moles of l i by avogadro's number to find the number of l i atoms. 7.1 one mole of a given substance is defined as the amount of substance w …. Use molar masses to convert masses to moles, and use avogadro's number (6.02×1023 particles per mole) to convert number of particles to moles. next, convert moles of the given reactant or product to moles of the desired reactant or product using the coefficients of the balanced chemical equation.

Solved х Learning Goal To Use Avogadro S Number To Convert Chegg Here’s the best way to solve it. multiply the number of moles of l i by avogadro's number to find the number of l i atoms. 7.1 one mole of a given substance is defined as the amount of substance w …. Use molar masses to convert masses to moles, and use avogadro's number (6.02×1023 particles per mole) to convert number of particles to moles. next, convert moles of the given reactant or product to moles of the desired reactant or product using the coefficients of the balanced chemical equation.
Comments are closed.