Understanding Enthalpy And Calorimetry Professor Dave Chegg Explain

Solved In Today's Workshop We Will Use Calorimetry To | Chegg.com
Solved In Today's Workshop We Will Use Calorimetry To | Chegg.com We'll take a look at enthalpy, heat capacity, specific heat, and how to measure enthalpy changes during chemical processes with calorimetry. Hess's law and enthalpy change | professor dave & chegg explain. in this video, @professordaveexplains teaches us how to calculate the amount of heat absorbed and released in a.
Solved Question 1 A Student Performs A Calorimetry | Chegg.com
Solved Question 1 A Student Performs A Calorimetry | Chegg.com What is heat? it's not just a movie with pacino and deniro. learn all about heat, and more importantly, enthalpy! energy exchange is a big part of chemistry . In this video, we're introducing the basics of thermochemistry, a specific area within thermodynamics focusing on heat transfer in chemical contexts. with th. How is it used to determine the enthalpy change in this experiment? 2. is enthalpy an extensive or intensive property. use the following equations to explain your choice: a >b, ah= 100 kj/mol; 1/2a >1/2b, ah= 50kj/mol. 3. specify the units for the following: a) heat of the reaction (q), b) enthalpy change (ah) 4. in part 1 for this. Join professor dave explains to run your own coffee cup calorimetry experiment, and gain a stronger understanding of how heat transfers between substances.

Solved Chemistry 261 Lab - Calorimetry And Enthalpy Of | Chegg.com
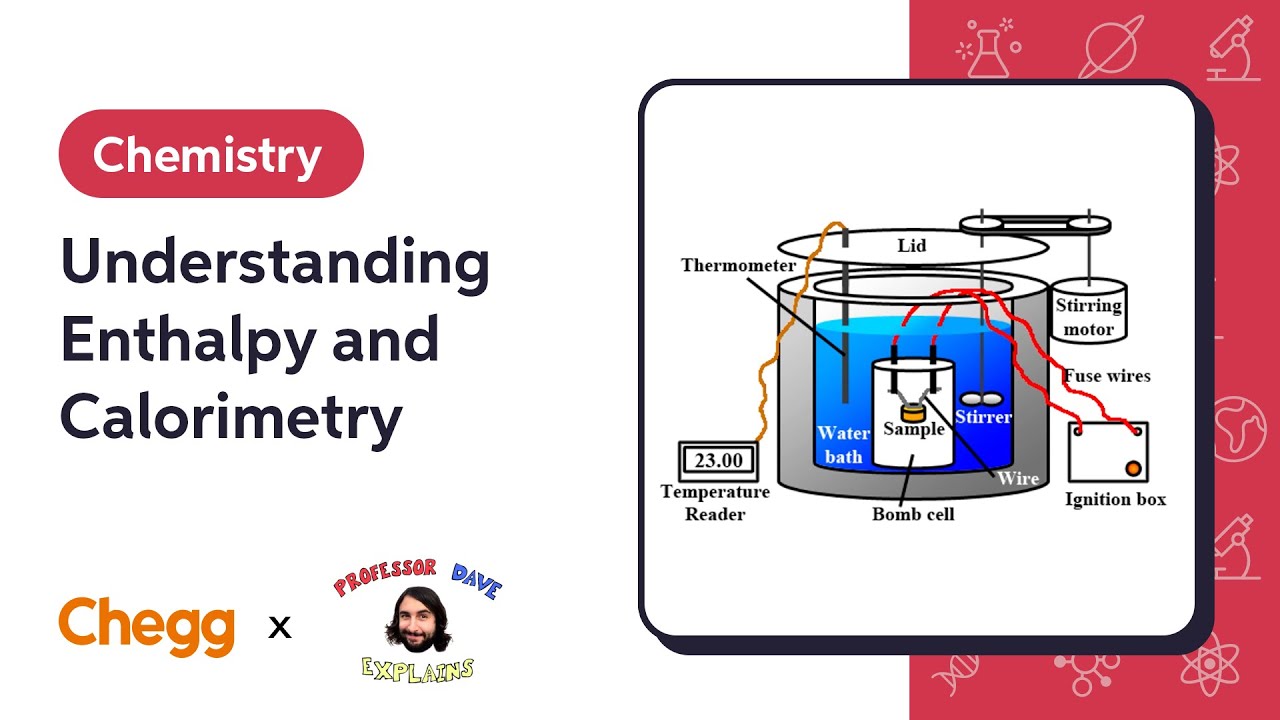
Solved Chemistry 261 Lab - Calorimetry And Enthalpy Of | Chegg.com How is it used to determine the enthalpy change in this experiment? 2. is enthalpy an extensive or intensive property. use the following equations to explain your choice: a >b, ah= 100 kj/mol; 1/2a >1/2b, ah= 50kj/mol. 3. specify the units for the following: a) heat of the reaction (q), b) enthalpy change (ah) 4. in part 1 for this. Join professor dave explains to run your own coffee cup calorimetry experiment, and gain a stronger understanding of how heat transfers between substances. Understanding these concepts helps predict reaction behavior and energy requirements in various chemical processes. hess's law states that the total enthalpy change for a reaction is independent of the pathway taken, allowing for the calculation of enthalpy changes through alternative reactions. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on the heat capacity of the system. We'll look at the definition of enthalpy of formation, see how it is useful for reactions that are dangerous or impractical to measure, and learn how to make calculations using tabulated data. Calorimetry is the experimental technique used to measure heat flow associated with chemical reactions. a calorimeter is the device used to measure temperature changes, which indicate heat absorbed or released. the value of Δh is determined by measuring temperature changes at constant pressure.
Solved Calorimetry Is A Method Used To Measure Enthalpy, Or | Chegg.com
Solved Calorimetry Is A Method Used To Measure Enthalpy, Or | Chegg.com Understanding these concepts helps predict reaction behavior and energy requirements in various chemical processes. hess's law states that the total enthalpy change for a reaction is independent of the pathway taken, allowing for the calculation of enthalpy changes through alternative reactions. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on the heat capacity of the system. We'll look at the definition of enthalpy of formation, see how it is useful for reactions that are dangerous or impractical to measure, and learn how to make calculations using tabulated data. Calorimetry is the experimental technique used to measure heat flow associated with chemical reactions. a calorimeter is the device used to measure temperature changes, which indicate heat absorbed or released. the value of Δh is determined by measuring temperature changes at constant pressure.
Understanding Enthalpy and Calorimetry | Professor Dave & Chegg Explain
Understanding Enthalpy and Calorimetry | Professor Dave & Chegg Explain
Related image with understanding enthalpy and calorimetry professor dave chegg explain
Related image with understanding enthalpy and calorimetry professor dave chegg explain
About "Understanding Enthalpy And Calorimetry Professor Dave Chegg Explain"











Comments are closed.